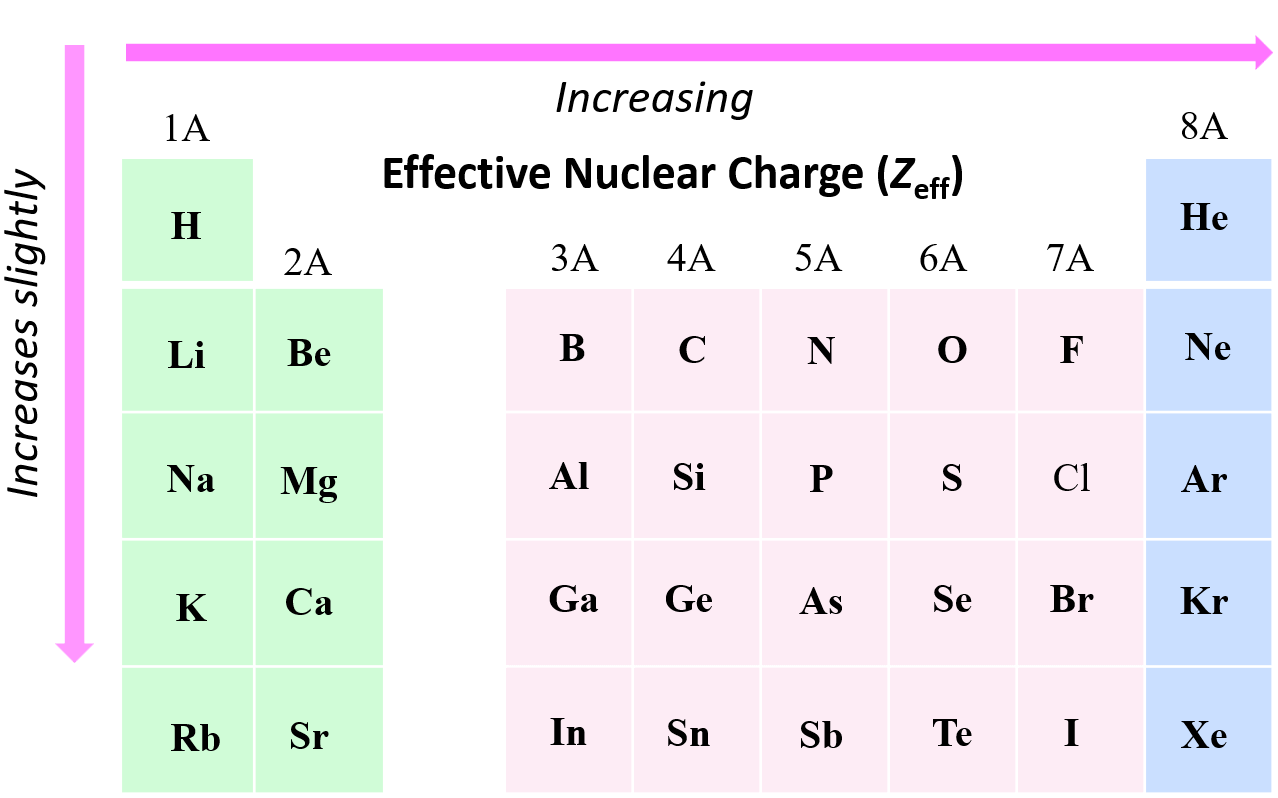
Define Electron Shielding And Effective Nuclear Charge . The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. This reduced charge is known as the effective. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,.
from general.chemistrysteps.com
in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. This reduced charge is known as the effective. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the.
Effective Nuclear Charge Chemistry Steps
Define Electron Shielding And Effective Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. This reduced charge is known as the effective. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the.
From www.youtube.com
How To Calculate The Effective Nuclear Charge of an Electron YouTube Define Electron Shielding And Effective Nuclear Charge This reduced charge is known as the effective. overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. effective nuclear charge, z eff, measures how strongly the. Define Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Atomic Electron Configurations and Chemical Periodicity Define Electron Shielding And Effective Nuclear Charge overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for. Define Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Chapter 8 Periodic Properties & Electron Configurations Bushra Define Electron Shielding And Effective Nuclear Charge overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. This reduced charge is known as the effective. the concept of electron shielding, in which intervening. Define Electron Shielding And Effective Nuclear Charge.
From list.ly
School college chemistry A Listly List Define Electron Shielding And Effective Nuclear Charge in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. This. Define Electron Shielding And Effective Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge Chemistry Tutorial YouTube Define Electron Shielding And Effective Nuclear Charge the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the. Define Electron Shielding And Effective Nuclear Charge.
From www.learner.org
Organizing Atoms and Electrons The Periodic Table Annenberg Learner Define Electron Shielding And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the. Define Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Define Electron Shielding And Effective Nuclear Charge This reduced charge is known as the effective. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. the concept of electron shielding, in which intervening. Define Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Unit 8 Periodic Properties of the Elements PowerPoint Define Electron Shielding And Effective Nuclear Charge The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. This reduced charge is known as the effective. the concept of electron shielding, in which intervening. Define Electron Shielding And Effective Nuclear Charge.
From gamma.app
Understanding Shielding Effect and Effective Nuclear Charge Define Electron Shielding And Effective Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. . Define Electron Shielding And Effective Nuclear Charge.
From www.vedantu.com
Shielding Effect and Effective Nuclear Charge Important Concepts for JEE Define Electron Shielding And Effective Nuclear Charge This reduced charge is known as the effective. overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. effective nuclear charge, z eff, measures how strongly. Define Electron Shielding And Effective Nuclear Charge.
From www.numerade.com
SOLVEDDefine shielding and effective nuclear charge. What is the Define Electron Shielding And Effective Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. This reduced charge is known as the effective. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. The shielding effect describes the balance between the pull of the protons on valence. Define Electron Shielding And Effective Nuclear Charge.
From www.youtube.com
nuclear charge and electron shielding YouTube Define Electron Shielding And Effective Nuclear Charge overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for. Define Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Effective Nuclear Charge PowerPoint Presentation, free download Define Electron Shielding And Effective Nuclear Charge overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from. Define Electron Shielding And Effective Nuclear Charge.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube Define Electron Shielding And Effective Nuclear Charge in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. This. Define Electron Shielding And Effective Nuclear Charge.
From slideplayer.com
Effective Nuclear Charge, Shielding, and Trends on the Periodic Table Define Electron Shielding And Effective Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons.. Define Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Periodic Properties of the Elements PowerPoint Presentation, free Define Electron Shielding And Effective Nuclear Charge overall, the outer electrons experience a lower force and a reduced nuclear charge due to shielding by the inner electrons. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. This. Define Electron Shielding And Effective Nuclear Charge.
From www.slideserve.com
PPT Effective nuclear charge, Z eff = Z S PowerPoint Presentation Define Electron Shielding And Effective Nuclear Charge Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the. in chemistry, the shielding effect sometimes referred to as atomic shielding or electron shielding describes the attraction between. The shielding effect describes the balance between the pull of the protons on valence electrons and the. Define Electron Shielding And Effective Nuclear Charge.
From socratic.org
How are shielding effect and atomic radius related? Socratic Define Electron Shielding And Effective Nuclear Charge This reduced charge is known as the effective. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. Effective nuclear charge is a measure of the net positive charge experienced by an electron in an atom, while the shielding effect is the. The shielding effect describes the balance between. Define Electron Shielding And Effective Nuclear Charge.